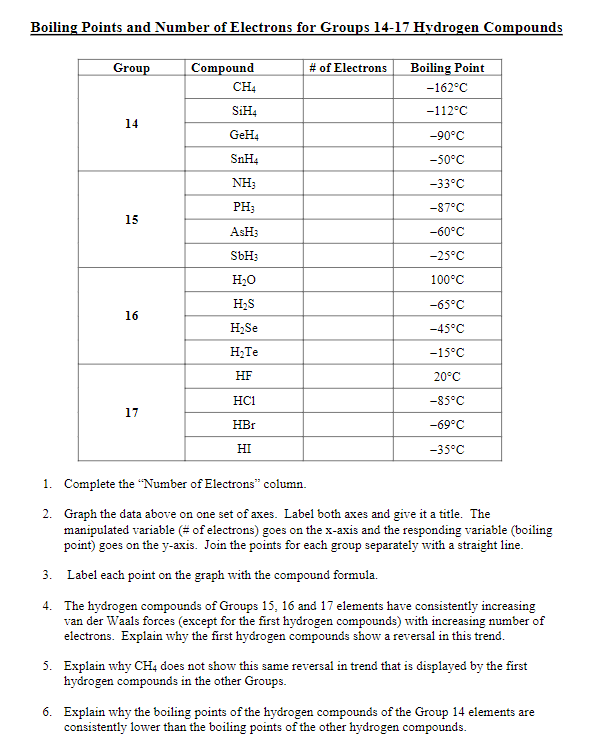
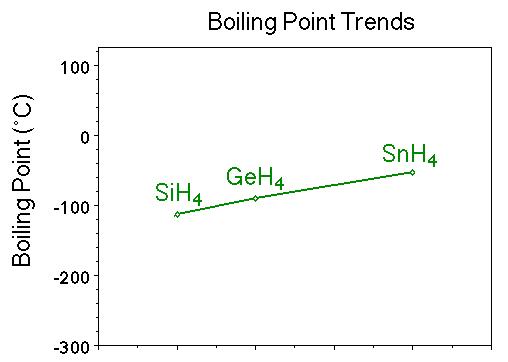
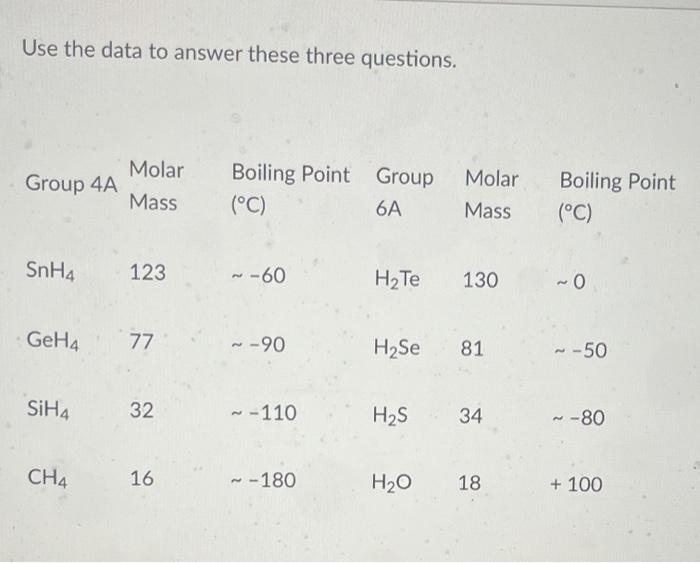
SOLVED:Estimate the missing boiling point in the following series of compounds. (a) CH4,-164^∘ C ; SiH4,-112^∘ C ; GeH4,-90^∘ C sn H4, ?^∘ C (b) H2 O, ?^∘ C ; H2 S,-61^∘
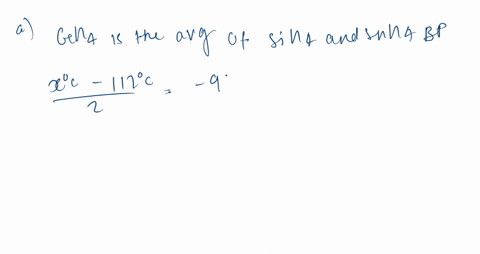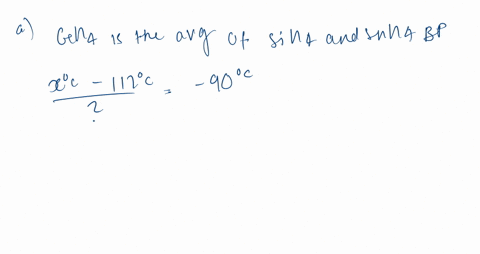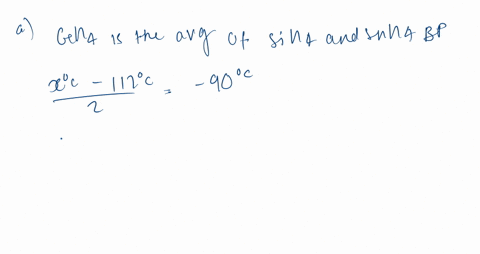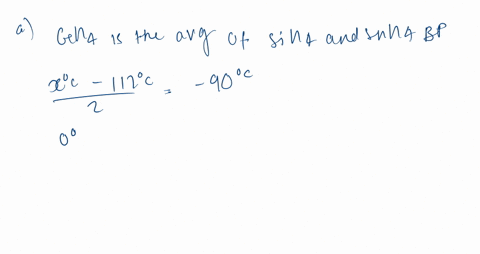
SOLVED:Estimate the missing boiling point in the following series of compounds. (a) CH4,-164^∘ C ; SiH4,-112^∘ C ; GeH4,-90^∘ C sn H4, ?^∘ C (b) H2 O, ?^∘ C ; H2 S,-61^∘
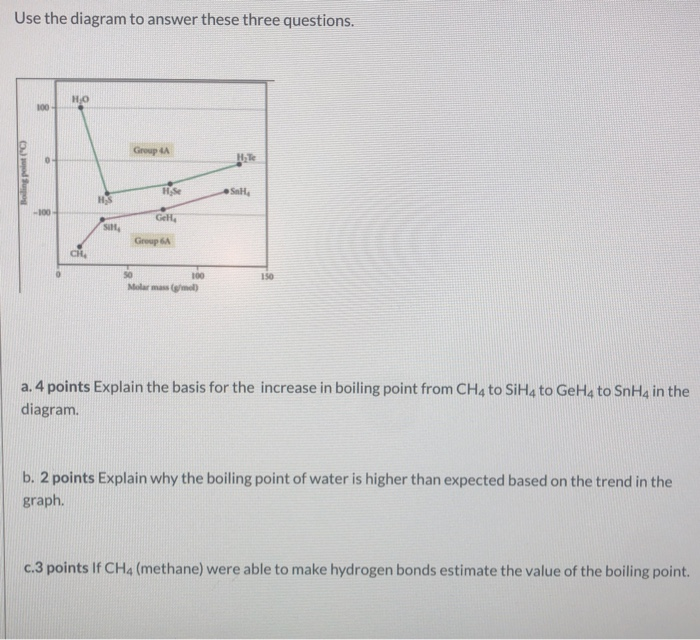
Consider the following: CH4, SiH4, GeH4, SnH4 The boiling points for these compounds increase roughly at the same rate except for CH4. Why does CH4 have a significantly lower boiling point than
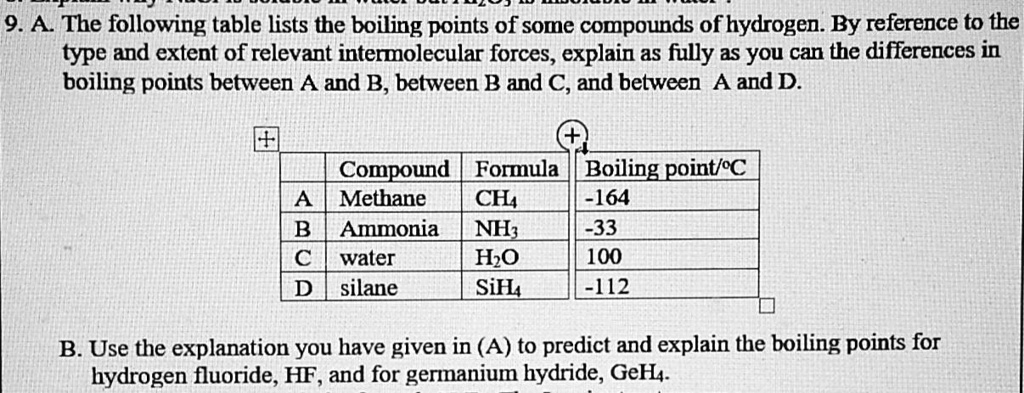
SOLVED: 9.A The following table lists the boiling points of some compounds ofhydrogen. By reference to the type and extent of relevant intermolecular forces, explain as fully as you can the differences
Consider the following: CH4, SiH4, GeH4, SnH4 The boiling points for these compounds increase roughly at the same rate except for CH4. Why does CH4 have a significantly lower boiling point than